
India plays an important role in the global pharmaceuticals and vaccine industry. It is the largest provider of generic medicines globally. The country has a share of 20% in the global supply volume and contributes to around 60% of the global vaccines. India ranks third in the world in terms of volume and is the fourteenth largest in terms of value. Key segments of the Indian pharmaceutical industry are OTC medicines, Generics, APIs, Vaccines, Biosimilars, and Custom Research Manufacturing (CRM).
India is the world leader in supplying vaccines like DPT, BCG, and Measles. It also has the highest number of US FDA-approved plants outside of the USA. The key USP of the Indian Pharmaceutical Industry is affordable price and high quality and because of this, India is also sometimes called the “Pharmacy of the World”. The total annual turnover of the industry was US$ 49.78 billion in FY23 and US$ 41.68 billion in 2021-22. One of the major achievements of the Indian Pharma Industry is access to affordable HIV drugs. Additionally, India is one of the largest suppliers of low-cost vaccines to the world.
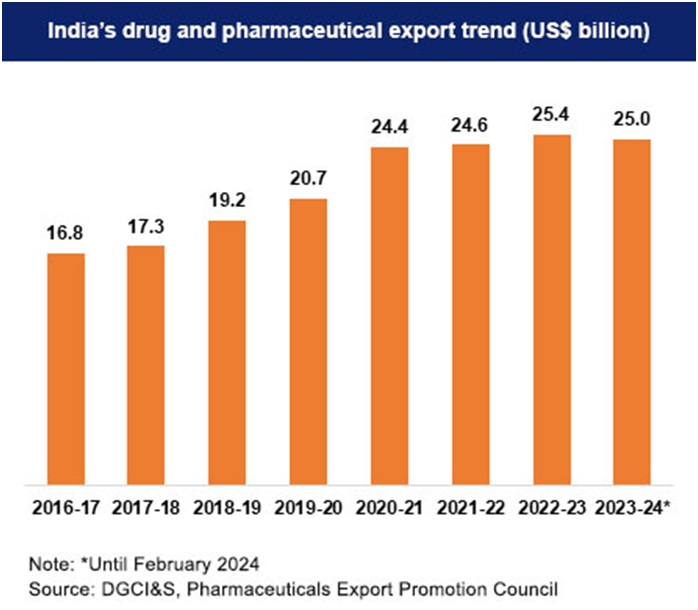
India majorly exports drug formulations & biologicals, and these products contribute to about 75% of the total pharmaceutical exports out of India.
Export Destinations
- USA
- BELGIUM
- SOUTH AFRICA
- BRAZIL
- NETHERLANDS
- RUSSIA
- FRANCE
Government Initiatives
The Government of India has set up several schemes to further strengthen the pharmaceutical industry. The Strengthening of Pharmaceutical Industry (SPI) scheme focuses on bolstering the existing infrastructure facility, with a total financial outlay of Rs. 500 crore (US$ 64.5 million).
The Production Linked Incentive (PLI) scheme supports domestic manufacturing of 41 critical pharmaceutical ingredients with a total outlay of Rs 6,940 crore (US$ 834 million) until 2029-30.
For the promotion of the Indian pharmaceutical industry, the Pharmaceutical Promotion and Development Scheme (PPDS) was introduced in 2017 with financial support for conducting seminars, conferences, exhibitions and delegations.
Pharmaceutical Technology Upgradation Assistance Scheme (PTUAS) was implemented to facilitate small and medium-sized enterprises (SMEs) to attain WHO-GMP norms. This will enable them to compete on a global scale.
The Pradhan Mantri Bhartiya JanaushadhiPariyojana’ (PMBJP), was originally launched in 2008 as the “Jan Aushadhi Scheme”. This scheme aims to make quality affordable generic medicines available to all.
Governing Body
Pharmaceutical Export Promotion Council of India (Pharmexcil)
Pharmexcil is a promotion body set up by the Government of India to promote the Indian pharmaceutical industry. The roles of the council are to advise the government, organize seminars and meetings on export-related issues, organize business meetings in India and abroad and organize trade delegations. The council also assists its members in getting Market Access Incentive (MAI) claims from the Government of India.
Department of Pharmaceuticals
The Department of Pharmaceuticals was formed in 2008 to focus on the development of the pharmaceutical sector in the country. The key functions of the department are to ensure the availability of drugs at reasonable prices, proper functioning of Central Pharma Undertakings, support projects and revival of schemes, ensure proper management, develop human resources and infrastructure, formulate schemes & projects and formulate an annual plan, budget and monitoring of budget expenditure.
Also Read This: A Detailed Report On Sundarja mango of Rewa Import-Export
Business Start Up Process
STEP 1 :- Business Registration
Proprietorship Firm
MSME (Udyam Aadhar)
Partnership Firm
Deed
ROF (Registration of Firm)
LLP (Limited Liability Partnership)
Deed
CIN (Certificate of Incorporation)
Company
MOA
AOA
ROC
STEP 2 :- PAN
Proprietor – Individual (Self)
Partnership Firm – Firm’s Name
LLP (Limited Liability Partnership) – LLP’s Name
Company – Company’s Name
STEP :- 3 GST (Goods & Services Tax)
STEP :- 4 Bank Account (Current A/c)
STEP :- 5 IEC – Importer Exporter Code
STEP :- 6 RCMC – Registration with Export Promotional Bodies (EPC, CB, DA, Etc.)
STEP :- 7 Port Registration (Nearest Port where you source)
ICEGATE Registration
STEP:- 8 COC – Chamber of Commerce
DGFT Registration
STEP: -9 Others – If Any
Documents Mandatory For Export
Pre-Shipment
a. Proforma Invoice / Agreement / Contract
b. Letter of Credit
Post-Shipment
a. Commercial Invoice
b. Packing List
c. B/L / AWB / LR
d. Certificate of Origin
e. Insurance
f. Others (if any)






